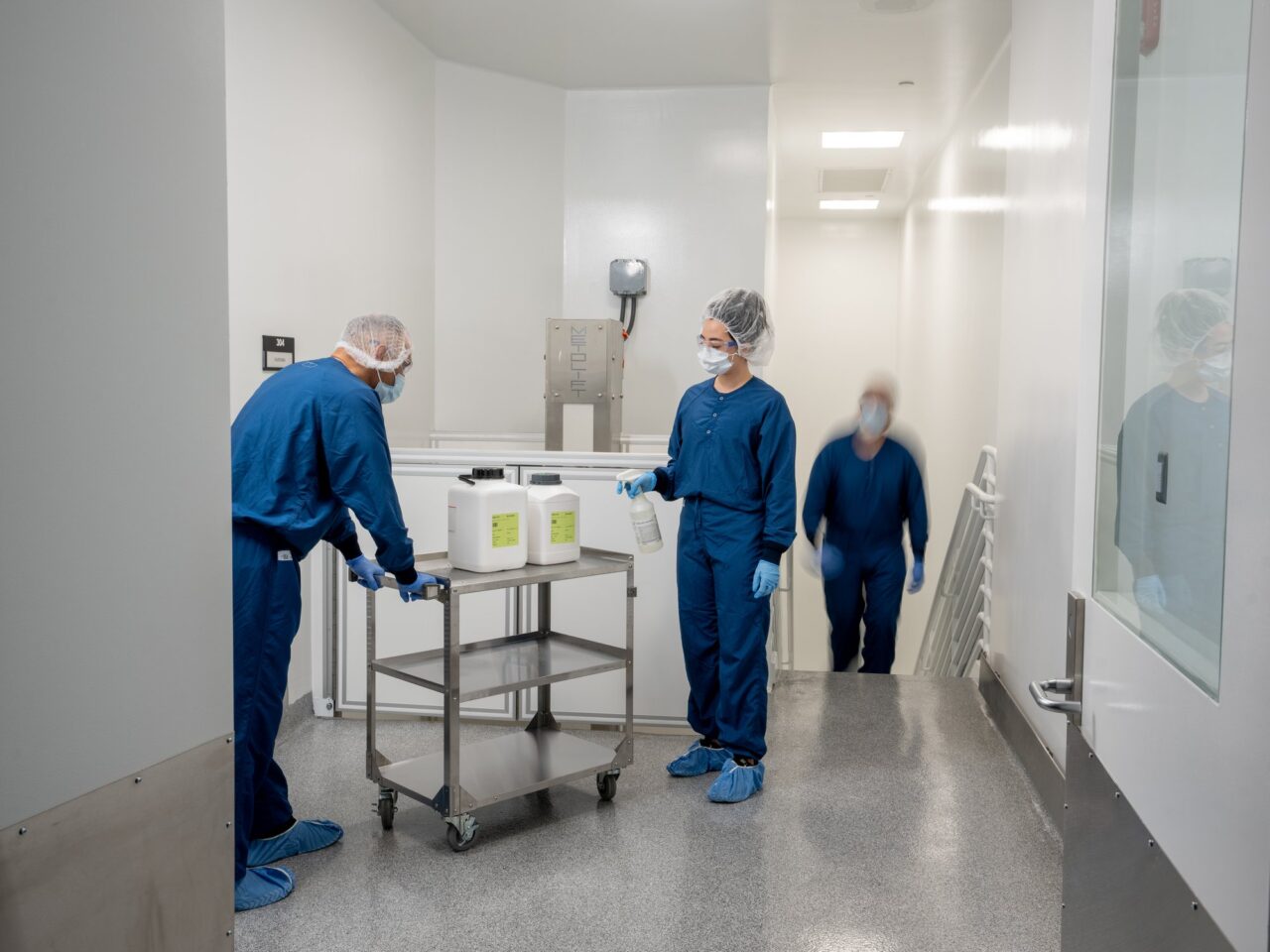
We Get You – We Understand that life science tools manufacturers providing biotechnology and advanced therapies end-customers with upstream and downstream process liquids require ever expanding cGMP manufacturing capabilities to successfully serve their end-customer’s demand for market leading product quality and customer experience.
Market – LifeScience Tools Manufacturers
Life Science Tools Manufacturers

Empowering the discovery, development and production of transformational therapies

GeminiBio enables life science tools manufacturers to significantly expand the scale – and flexibility – of their cGMP process liquid manufacturing.
GeminiBio has two manufacturing facilities, both located in West Sacramento, CA, comprising a total of 57,000 square feet. To meet the stringent needs of biotechnology and life science tools customers, the company’s cell culture sera and bioprocess liquid manufacturing facilities are segregated between animal origin-free cGMP manufacturing and animal component cGMP manufacturing.
Life science tools manufacturers that work with GeminiBio are better positioned to meet demanding end-customer requirements, while simultaneously optimizing the productivity of their existing self-manufacturing assets.
GeminiBio’s cGMP process liquid contract manufacturing capabilities, which include cGMP batch sizes from 10-liters to 10,000-liters and ability to fill into diverse container types (rigid and flexible) and sizes (500 mL bottles to 1,000-liter pallet tanks), allow tools manufacturers to optimize their market penetration and end-customer satisfaction. In particular, our capabilities enable companies to more effectively serve the plasmid DNA and viral vector manufacturing requirements of cell and gene therapy end-customers and CDMOs.

Our Integrated Quality Approach – Our commitment to the Life Science Tools Market
Quality

How Can We Help?
Select from drop down
Contact: United States Sales Team
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America